Abstract
Introduction: Standard of care treatments for patients (pts) with newly diagnosed multiple myeloma (NDMM) include triplet regimens containing a proteasome inhibitor (PI) or an immunomodulatory drug (IMiD), with or without autologous stem cell transplant (ASCT). Among triplets, extended treatment with KRd has emerged as a highly active combination in NDMM (Jakubowiak AJ, et al. Blood, 2012;120[9]:1801-1809). As multiple myeloma progresses, the depth and duration of clinical response is reduced with each treatment relapse, making it critical for front-line therapy to drive pts to deep and sustained clinical responses. Daratumumab (DARA) is a human IgGκ monoclonal antibody (mAb) targeting CD38 with direct on-tumor and immunomodulatory mechanisms of action. DARA induces rapid, deep, and durable responses in combination with dexamethasone and either bortezomib or an IMiD (lenalidomide or pomalidomide) in pts with relapsed/refractory MM (RRMM; Palumbo A, et al. NEJM . 2016 375[8]:754-766; Dimopoulos MA, et al. NEJM . 2016 375[14]:1319-1331; Chari A, et al. Blood . 2017; epub, ahead of print). This open-label, multicenter, phase 1b study investigated the safety profile and efficacy of DARA in combination with KRd in NDMM.
Methods: Pts with NDMM were enrolled regardless of transplant eligibility, and had no evidence of clinically significant cardiac disease. Pts received a split first-dose of daratumumab (8 mg/kg on Days 1 and 2 of Cycle 1) and received 16 mg/kg weekly for the remainder of Cycles 1 and 2, every 2 weeks on Cycles 3-6, and every 4 weeks thereafter. Carfilzomib (K) 20 mg/m2 was administered on Cycle 1 Day 1 and escalated to 70 mg/m2 on Cycle1 Day 8, and was administered weekly on Days 1, 8, and 15 of each cycle. Pts received lenalidomide 25 mg on Days 1-21 of each cycle and dexamethasone at a dose of 40 mg/week. Pts were treated for ≤13 treatment cycles or until elective discontinuation for ASCT. Primary endpoints included safety and tolerability; overall response rate (ORR), duration of response, time to response, infusion-related reactions (IRR), and progression-free survival (PFS) were also examined. For DARA interference on serum immunofixation (IFE), a second reflex assay using an anti-idiotype mAb was used to confirm DARA migration on the IFE.
Results: Among the 22 pts enrolled in the study, the median (range) age was 59.5 (34-74) years and 95% of pts had an ECOG score ≤1. After median (range) duration of follow-up of 13.1 (6.6-14.9) months, pts received a median (range) of 12 (1-13) treatment cycles. Treatment discontinuations were due to elective ASCT (n = 6), adverse events (AE; n = 1), and progressive disease (n = 1).
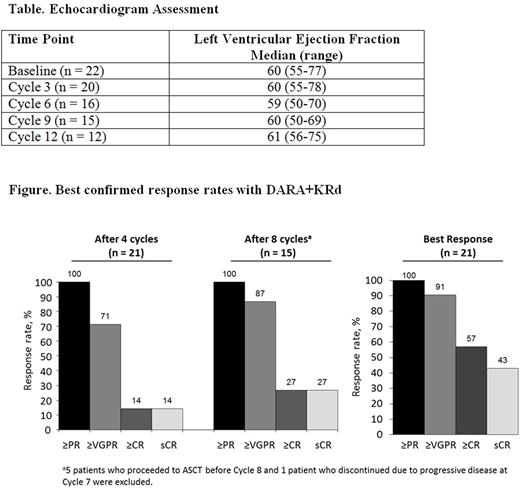
At the clinical cutoff date of June 16, 2017, the most common (>10%) grade 3/4 treatment emergent AEs (TEAEs) included lymphopenia (14 [64%]), neutropenia (4 [18%]), diarrhea (4 [18%]), and pulmonary embolism (3 [14%]). Serious TEAEs occurred in 10 (46%) pts, with pulmonary embolism being the most common (3 [14%]). A transient grade 3 cardiac failure was reported in 1 pt who resumed treatment on Cycle 2 Day 1 with a reduced K dose (56 mg/m2). IRRs (all grade 1 or 2) were reported in 27% of pts and occurred primarily during the first infusion. Median left ventricular ejection fraction did not change over time from baseline (Table).
21 response-evaluable pts achieved an ORR of 100%, including 43% stringent complete response (sCR), 14% complete response (CR), 33% very good partial response (VGPR), and 10% partial response (PR); responses deepened with treatment duration (Figure). Responses among 15 pts who did not undergo ASCT were as follows: 40% sCR, 20% CR, 27% VGPR, 13% PR. At the time of follow-up, all pts were alive and the 12-month PFS rate was 95% (95% confidence interval, 70-99). CD34+ cell collection yields were consistent with previous KRd studies (median, 10.6 x 106 cells/kg) and 15/20 eligible pts had a response of ≥VGPR prior to stem cell harvest. Among the 20 eligible pts, median (range) number of cycles received prior to stem cell harvest was 5 (4-9).
Updated safety and efficacy data will be presented.
Conclusion: DARA+KRd, with weekly K dosing, is well tolerated in pts with NDMM and demonstrates a safety profile consistent with previous reports of DARA and KRd. High response rates were observed with DARA+KRd and the depth of response improved with treatment duration. No adverse impact on stem cell collection was observed. These data support further investigation of DARA+KRd in NDMM.
Chari: Array BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; Biotest: Other: Research funding (to AC's institution); Onyx: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Pharmacyclics: Research Funding; Bristol-Myers Squibb: Consultancy, Other: Research funding (to AC's institution); travel; Acetylon Pharmaceuticals: Other: Research funding (to AC's institution). Usmani: Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Array BioPharma: Honoraria, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Onyx: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pharmacyclics: Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Speakers Bureau; Skyline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Millennium: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Honoraria, Research Funding; Novartis: Speakers Bureau. Krishnan: Janssen: Consultancy, Speakers Bureau; Takeda: Speakers Bureau; Celgene: Consultancy, Equity Ownership, Speakers Bureau; Onyx: Speakers Bureau; Sutro: Consultancy. Comenzo: Janssen, Prothena, Takeda, Karyopharm: Research Funding; Janssen, Prothena: Consultancy, Research Funding. Wu: Janssen: Employment. Wang: Janssen: Employment. Doshi: Janssen: Employment. Weiss: Prothena: Honoraria; Janssen: Honoraria; Prothena: Research Funding; Alnylam: Honoraria; Janssen: Research Funding. Schecter: Janssen: Employment. Jakubowiak: Amgen Inc., BMS, Celgene, Janssen, Karypharm, Millennium-Takeda, Sanofi, SkylineDX: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; University of Chicago: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal